Published February 2021
While the beginning distribution phases of the COVID-19 vaccines are spreading hope and optimism for some, questions around everything from speed to market to efficacy and side effects are still swirling in the minds of many. Is it safe? Should I get it? If so, when can I get it?
With expert guidance from Dr. Patricia Toro, MD, MPH, infectious disease specialist and other trusted medical sources, we’ve sought to help answer these pressing questions and ease concern.
Here’s what you need to know right now about the COVID-19 vaccine development, safety and efficacy:
1. What is the COVID-19 vaccine?
By definition, a vaccine is a substance that helps to protect against and prevent certain illnesses. Currently, there are two types of vaccines available for COVID-19, messenger RNA (mRNA) vaccines that include the Pfizer-Biotech and Moderna vaccines, and a viral vector vaccine, made by Johnson & Johnson:
mRNA Vaccines
mRNA vaccines have been developed and implemented for earlier coronaviruses like SARS and MERS. Instead of putting weakened or activated germs in the body like traditional vaccines, the mRNA vaccine teaches cells how to make a protective protein. Should the real virus enter the body, that new protein triggers an immune response that produces antibodies and prevents us from getting sick.
Viral Vector Vaccines
Viral vector vaccines piggyback the instructions to fight the coronavirus onto a separate and different virus, called an adenovirus. The virus is not alive and therefore cannot infect the individual receiving the vaccination. Viral vector vaccines have already been used to fight a number of diseases, including Zika, influenza viruses, HIV, malaria and Ebola.
Both Vaccines Had a Blueprint
The existing “blueprints” from previous vaccines enabled vaccine manufacturers to develop the COVID-19 vaccine more quickly. Dr. Toro notes that this was one of several reasons they were able to develop these vaccines in “10 months versus 10 to 15 years.”
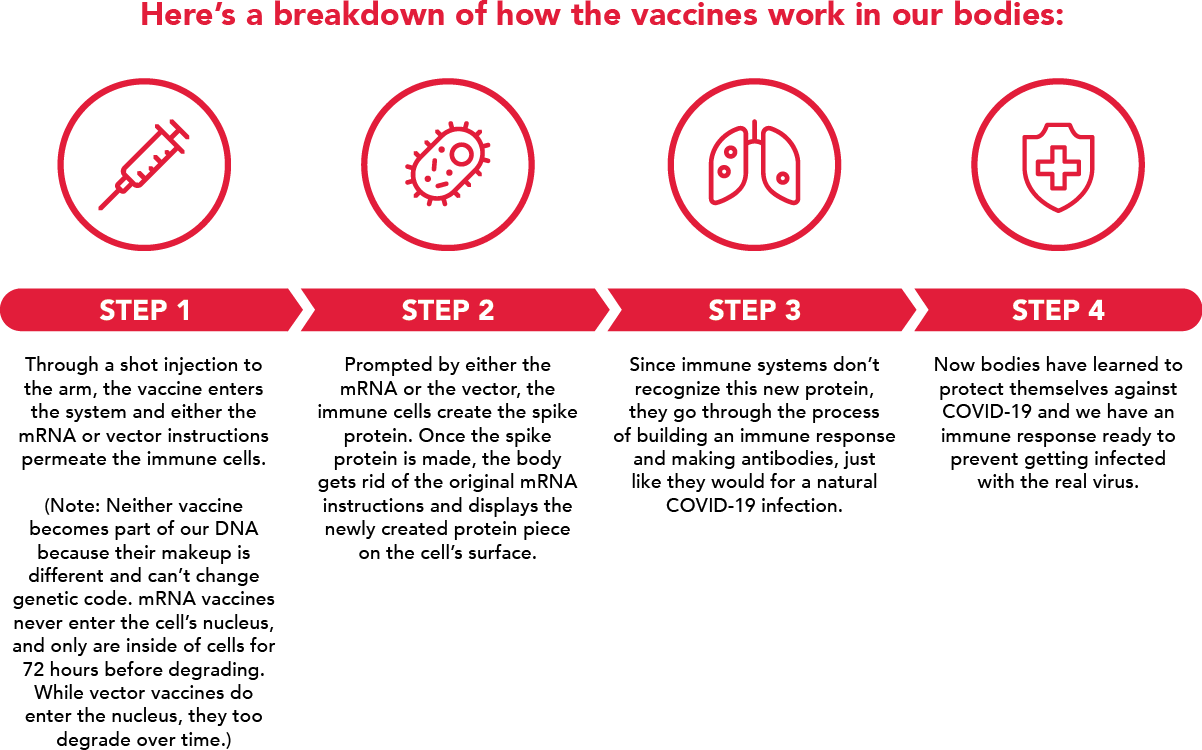
2. How do they work?
Both types of vaccines help create a protein in the body. More specifically, the COVID-19 vaccines instruct our cells to create the “spike protein”–a protein found on the surface of the virus that causes COVID-19.
*Sources:
https://www.gavi.org/vaccineswork/will-mrna-vaccine-alter-my-dna [gavi.org]
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html [cdc.gov]
3. How were they developed and authorized?
All vaccines are required to go through extensive testing before they’re approved or authorized for emergency use by the U.S. Food and Drug Administration (FDA) and introduced to the public. With prioritized funding and resources, and trials running in parallel, the COVID-19 vaccines went through the same clinical development process as all other new vaccines, just in a much shorter time frame. The World Health Organization breaks down new vaccine development as follows:
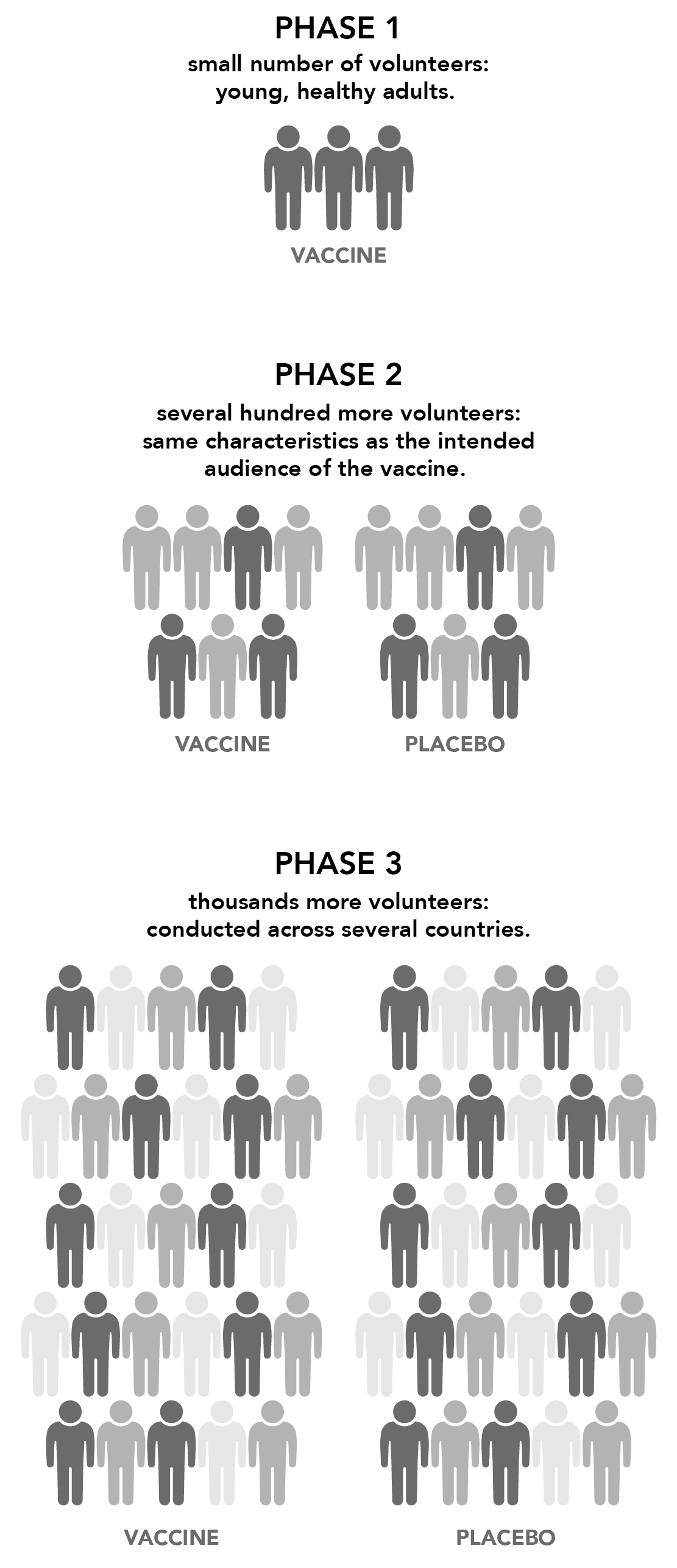
The vaccine is administered to a small number of volunteers to test for 1) safety, 2) efficacy (does it generate an immune response?) and 3) the appropriate dosage. In this phase, vaccines are typically tested on young, healthy, adult volunteers.
Then the vaccine is given to several hundred more volunteers to further determine its safety and ability to generate an immune response. Participants in this phase generally have the same characteristics (such as age and sex) as the intended audience of the vaccine, and there are usually multiple trials to evaluate different age groups and formulations of the vaccine. At this stage, a control group (a group that gets a placebo instead of the real vaccine) is added to determine if changes in the vaccinated group are truly caused by the vaccine or occurred by chance.
Next, the vaccine is administered to thousands more volunteers and compared to a control group to determine effectiveness and safety in a larger number of volunteers. Moderna enrolled about 30,000 volunteers while Pfizer enrolled a little more than 43,000, numbers Dr. Toro notes are typical for a Phase 3 trial. These trials are often conducted across several countries and sites within a country to confirm that the vaccine performance findings apply to different populations. Every Phase 3 study is reviewed by the Data Safety Monitoring Board, so that the clinical trial is stopped should the participants be harmed by the vaccine. For all of these vaccines, the Phase 3 studies were completed and safety was maintained to the end.
4. Should I get vaccinated?
The CDC reports that current data shows the vaccines are highly effective. In every Phase 3 study for these vaccines, no individuals died of COVID-19 if they received the vaccine, which is a key metric of effectiveness. The mRNA vaccines prevented any kind of symptomatic infection about 95% of the time. Meanwhile, The Johnson & Johnson vaccine prevented any symptomatic infection 72% of the time in the U.S.
Getting vaccinated can protect you, and early evidence suggests that vaccination may decrease your risk of transmitting the virus if you are infected.
So, is it better to receive the mRNA vaccines?
No. The best vaccine to take is the one that’s available to you the soonest.
There are two contributing factors as to why the Johnson & Johnson vaccine results differ from the mRNA vaccine results:
- The Johnson & Johnson vaccine was tested in different countries than the mRNA vaccines, with different populations. In particular, it was tested in Brazil and South Africa while the variant coronaviruses were the dominant strains. The mRNA vaccines were not tested against variants.
- The Johnson & Johnson vaccine was also tested at a different time during the epidemic. The background transmission rate may have impacted the final efficacy results. On the most important metric, preventing illness so bad that an individual is either hospitalized or dies, the Johnson & Johnson vaccine was successful 85% of the time. And again, no one who received this vaccine died in the Phase 3 trial.
5. When can I get vaccinated?
While COVID-19 vaccines are currently available in all states, availability continues to be limited and priority groups’ eligibility is still being determined by each individual state. Use the following resources to stay in the know on the coronavirus vaccine timeline in your state:
6. How many shots do I need to be fully vaccinated?
There are currently three vaccines authorized for emergency use in the U.S. Two of these vaccines, created by Moderna and Pfizer-Biotech, require two shots that will need to be taken at least a few weeks apart for maximum protection. The third vaccine from Johnson & Johnson requires only a single shot. These vaccines protect you fully approximately two weeks after receiving the final dose.
Once you receive the vaccine, the CDC has developed a smartphone app called v-safe that you can download to help monitor your health and remind you when you’re due for your second dose.
The CDC recently shared updated, less restrictive guidelines stating that individuals who have been fully vaccinated can:
- Visit with other fully vaccinated individuals indoors without wearing masks or physical distancing.
- Visit with unvaccinated individuals from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing.
- Refrain from quarantine and testing following a known exposure if asymptomatic.
If you are in public, however, everyone should continue to wear a mask and continue physical distancing. And anyone who has symptoms should continue to be tested, no matter their vaccination status.
7. Are the vaccines safe? Are there side effects?
These vaccines are safe. Just like with any vaccine, COVID-19 vaccine side effects can occur as your body starts to build protection and can include swelling or pain at the site of the shot, fevers, chills, tiredness, nausea or headaches. These symptoms may last one to two days and are generally mild. This shows that the vaccine is working in your body.
Very rarely (about four times in one million injections) someone has a severe reaction to the vaccine. Out of caution for this, you may be asked to stay at the vaccination site for 15 to 30 minutes after your vaccine to be monitored. Any reaction can be easily treated by on-site health care providers.

After you’ve been vaccinated, you can use the CDC’s v-safe app to get personalized health check-ins and report any side effects. If needed, a CDC professional will follow up to get additional information.
8. What about new strains or “variants?” Will a vaccine protect me against them?
Yes. The Johnson & Johnson vaccine was specifically tested against the B.1.351 (“South African”) and P.2 (“Brazil”) strains and proved to be very protective. The Pfizer and Moderna vaccines are being tested more formally against these “variants of concern,” and we hope to know more soon. That said, communities that have been hit hard by these variants display decreased infections and hospitalizations when they are vaccinated by the mRNA vaccines.
Dr. Toro further explains that “if the variants mutate more and the vaccines become ineffective, then these mRNA vaccines can be ‘retooled’ within six to eight weeks to work specifically against the variants.” A “booster” shot against these variants is currently being tested for the mRNA vaccines.
9. How long will the vaccines last?
Since the vaccines are so new, it’s unclear how long they will last until more data becomes available. That said, early data suggests that most people will be protected for at least eight months, and perhaps longer.
While there’s a lot we now know and can prepare for with the COVID-19 vaccines, there is still so much that’s consistently changing. Over the next few months, remember to stay aware of updates from your individual state, as well as the CDC for new developments and changes in availability. And remember that we are still in a pandemic, so for the protection of yourself and others, wear a mask and keep up the social distancing.
Update as of April 13, 2021: The U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) have recommended a pause in the use of the Johnson & Johnson (J&J) COVID-19 vaccine. The agencies are reviewing data tied to six cases of a rare and severe type of blood clot in combination with low levels of blood platelets among individuals who received the J&J vaccine. While these events seem to be extremely rare, the CDC and FDA are recommending a pause in using the J&J vaccine out of an abundance of caution. Please contact your health care provider if you have received the J&J vaccine within the last three weeks and develop severe headache, abdominal pain, leg pain or difficulty breathing. We will continue to monitor and provide updates as the situation evolves. Please visit the CDC’s website for additional information.
Keep a pulse on the health trends that matter today.
To confirm eligibility for any programs or services mentioned in this article as it relates to your specific health plan, please reach out to your account executive or HR benefits team. You may also speak to our member services team at (888)-333-4742 or by sending a secure email. And for plan details and other member resources, log in to the member portal.